GRASSP Version 1
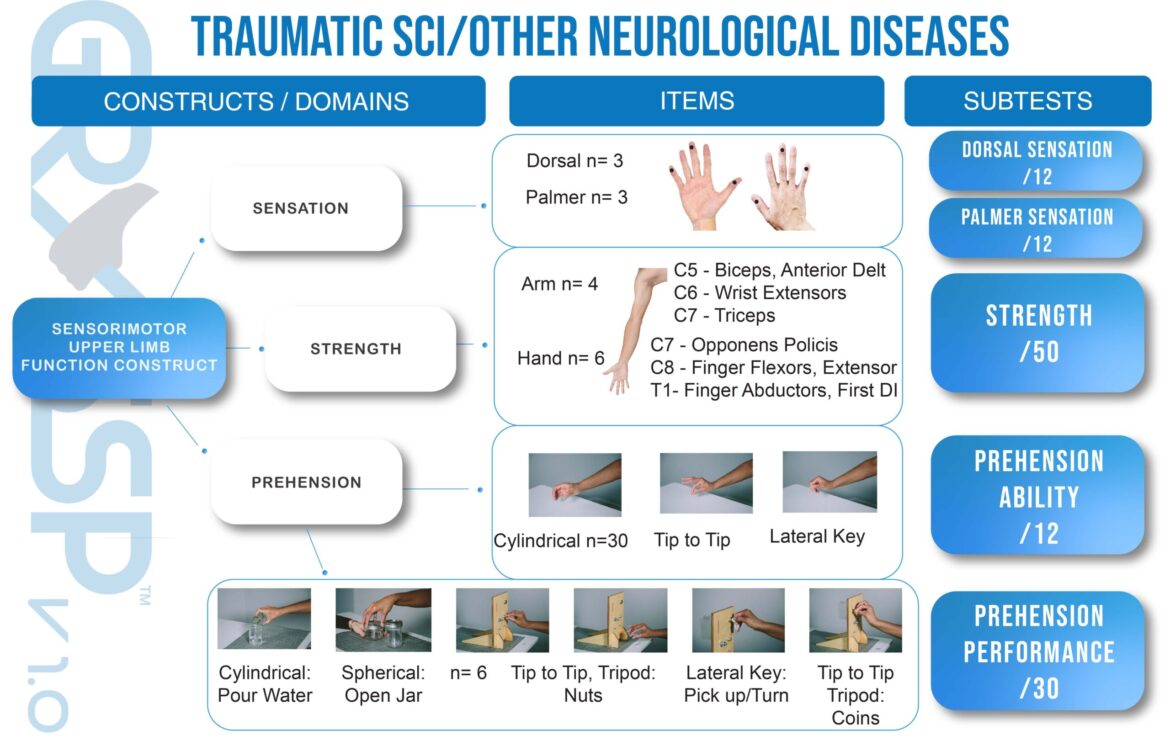
The Graded Redefined Assessment of Strength, Sensation and Prehension (GRASSP).
PURPOSE OF THE MEASURE
The GRASSP V1 was developed with the intent to be a clinical and a
research tool that would:
- capture information on upper limb impairment from the traumatic tetraplegic population
- obtain integrated sensory and motor impairment data, and discriminate the population according to impairment and function
- be responsive (sensitive) to change over time
- assess the extent of spontaneous (natural) recovery
- be applied in clinical settings and in clinical trials/studies to evaluate the effect of novel interventions
RECOMMENDED USE
The GRASSP is recommended for use in the early acute phase to any point in the post-injury time-course, particularly when a change in neurological status is the construct of interest. The GRASSP is intended for use with traumatic cervical SCI patients that are being followed for recovery of the upper limb. The GRASSP V1 is not designed for the non-traumatic SCI populations. Some work has been done that makes recommendations for GRASSP V1 use in the pediatric population. Currently there is some field testing being conducted to determine how GRASSP can be used with the tendon transfer/peripheral nerve transfer surgery groups.
The GRASSP measure is for use by clinicians in the clinical setting as a clinical outcome measure, researchers in the clinical/research setting as a primary or secondary outcome measure, and academics who are involved in investigator driven research. The developers and funding agencies that have supported GRASSP development would like to promote widespread use in both clinical and research settings. If you are interested in purchasing a GRASSP Kit please go to Products and Purchasing tab now.
VERSIONS of GRASSP
In 2009 GRASSP Version 1 was launched; currently there are three versions available:
GRASSP Version 1 – An impairment measure specific to the upper limb post traumatic tetraplegia.
GRASSP Version 2 – An impairment/activity measure specific to the upper limb post traumatic tetraplegia.
GRASSP Version Myelopathy – An impairment measure specific to the upper limb post non-traumatic tetraplegia secondary to cervical myelopathy related to tumor compression, epidural abscess or degenerative cervical myelopathy.
The original version is GRASSP Version 1, which is the version currently in use worldwide. Recently the GRASSP Research and Development Group (GRDG) has launched Version 2 and Version Myelopathy.
WHICH VERSION TO USE
GRASSP V1 is the original version developed and validated in 2009. This is the most robust of the three assessments. The most meaningful application of this measure is when the assessor is looking for more detailed information regarding the hand. GRASSP V1 is specifically developed to assess the traumatic tetraplegic upper extremity. GV1 is currently being used an important endpoint in many neuro-restorative trials where the hand is the primary target for treatment.
GRASSP V2 is a reduced version of GV1. This measure has stronger reliability for the subtests, and the sensitivity of the prehension performance is not as strong as GV1. Again, GV2 is specific to the traumatic tetraplegic upper limb and is currently being used as an important endpoint in many clinical drug trials where the therapy has a more centrally based target.
GRASSP VM is a reduced and modified version of GV1. It has measurement properties established, both reliability and validity. The application of this measure is specific to the non-traumatic tetraplegic upper limb. The emphasis is on manual dexterity, which distinguishes it from GV1 and GV2. GVM is useful where individuals have relatively intact function that is impaired due to slow and progressive compression of the cervical cord.
Translations available for GRASSP Versions:
GRASSP Manuals are available in select languages, translated GRASSP manuals are provided upon request only, please contact timryan@neuraloutcomes.com for more information. Translated manuals are only provided with purchase of a kit.
GV1 is available in English and Canadian French.
GV2 is available in English, German, Italian, Czech and Spanish.
GVM is available in English Only.
DISCLOSURE
GRASSP ver. 1, 2, and Myelopathy are non-medical, outcome measuring, and manually operative devices. These devices do not require any electric, electronic, chemical, magnetic power nor do they need any intravenous administration to operate. Since its design, manufacturing, packaging, and usage are known to be non-hazardous and safe for its users and administrators, the device is exempted from a CE or FDA certification.
For further information please refer the websites below:
https://www.nibusinessinfo.co.uk/content/products-need-ce-marking
http://tradecommissioner.gc.ca/world-monde/133383.aspx?lang=eng#Find
Although measuring equipment is mentioned as a category of products requiring CE certification, this refers to products that require calibration and/or are electronically powered. Again, the GRASSP or parts do not fall into these categories.