RESEARCH AND CLINICAL UTILITY
The GRASSP is currently being used worldwide in 9 countries and in multiple SCI centres.
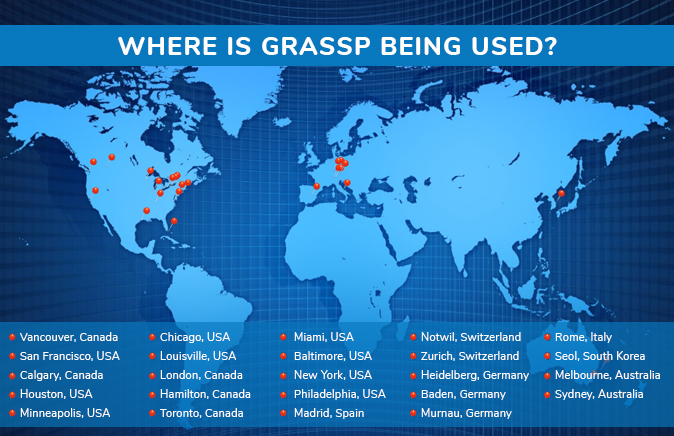
In addition to use in clinical settings around the world the GRASSP V1 is being implemented as a secondary endpoint in a number of investigator driven studies and sponsored clinical trials.
Investigator Driven Studies (Rehabilitation Studies)
- Pediatric Multi-Center Evaluation of Notable SCI Outcomes Instruments, Funded by Craig H. Neilsen, PI – MJ Mulcahey, Philadelphia PA
- Responsiveness of the Capabilities of Upper Extremity Test (CUE-T) Funded by Craig H. Neilsen, PI – Ralph Marino, Philadelphia
Investigator Driven SCI Trials and Studies
- Riluzole in Acute Spinal Cord Injury Trial (RISCIS)
- Nogo – Antibody Trial (NISCI Study) – Antibodies against Nogo-A to enhance plasticity, regeneration and functional recovery after acute spinal cord injury
Sponsored Driven SCI Trials and Studies
- Stem Cells Inc. Pathway Study – Single-Blind, Randomized, Parallel Arm, Phase II Proof-of-Concept Study of the Safety and Efficacy of HuCNS-SC Transplantation in Cervical Spinal Cord Injury
- Asterias Biotherapeutics, Star Study – Phase 1/2a Dose Escalation Study of AST-OPC1in Subjects with Cervical Sensorimotor Complete Spinal Cord Injury
- Vertex Pharmaceuticals, SPRING Study – Phase 2b/3, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Efficacy and Safety of VX-210 in Subjects With Acute Traumatic Cervical Spinal Cord Injury
- InVivo Therapeutics – Pilot Study of Clinical Safety and Feasibility of the Neuro-Spinal ScaffoldTM for the Treatment of Complete (AIS A) Traumatic Acute Spinal Cord Injury at the C5-T1 Neurological Levels